Unraveling the reaction mechanisms of water contaminants with an electrode surface during electrochemical oxidation
Overview
Per- and polyfluoroalkyl substances (PFASs) are very stable synthetic chemicals that are resistant to heat and repel water, oil, and stain. They are used in numerous commercial and industrial products including non-stick cookware, paints, polishes, clothes, cleaning products, food packaging, and fire-extinguishing foams. These chemicals are water soluble and resistant to biological degradation, and thus they have accumulated in the environment. Human exposure of PFASs can occur through many sources, which include drinking water, surface or ground water, seafood from contaminated water, food packaging, and seepage from areas where fire-extinguishing foams have been used. Both animal and human medical studies suggest that continuous exposure to PFASs could result in adverse health effects. As such, PFAS contamination is a critical environmental problem, which has prompted the Environmental Protection Agency to issue a Health Advisory Level of 0.024 ng/L for the combined concentrations of the two most prevalent PFASs (perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS)). Given the health concerns and increasingly lower regulatory standards, reliable low-cost methods for rapid monitoring and remediation are greatly needed.
We are trying to identify and mechanistically understand the reaction mechanisms of PFAS degradation in electrochemically destructive technologies. This approach will provide a fundamental and holistic understanding of: (1) how these technologies work for PFAS-impacted aqueous streams, (2) how applied voltage, varying PFAS concentration, electrolyte composition, co-occurring chemicals, and pH impact these technologies functionality and treatment effectiveness, and (3) what the limitations of these technologies are.
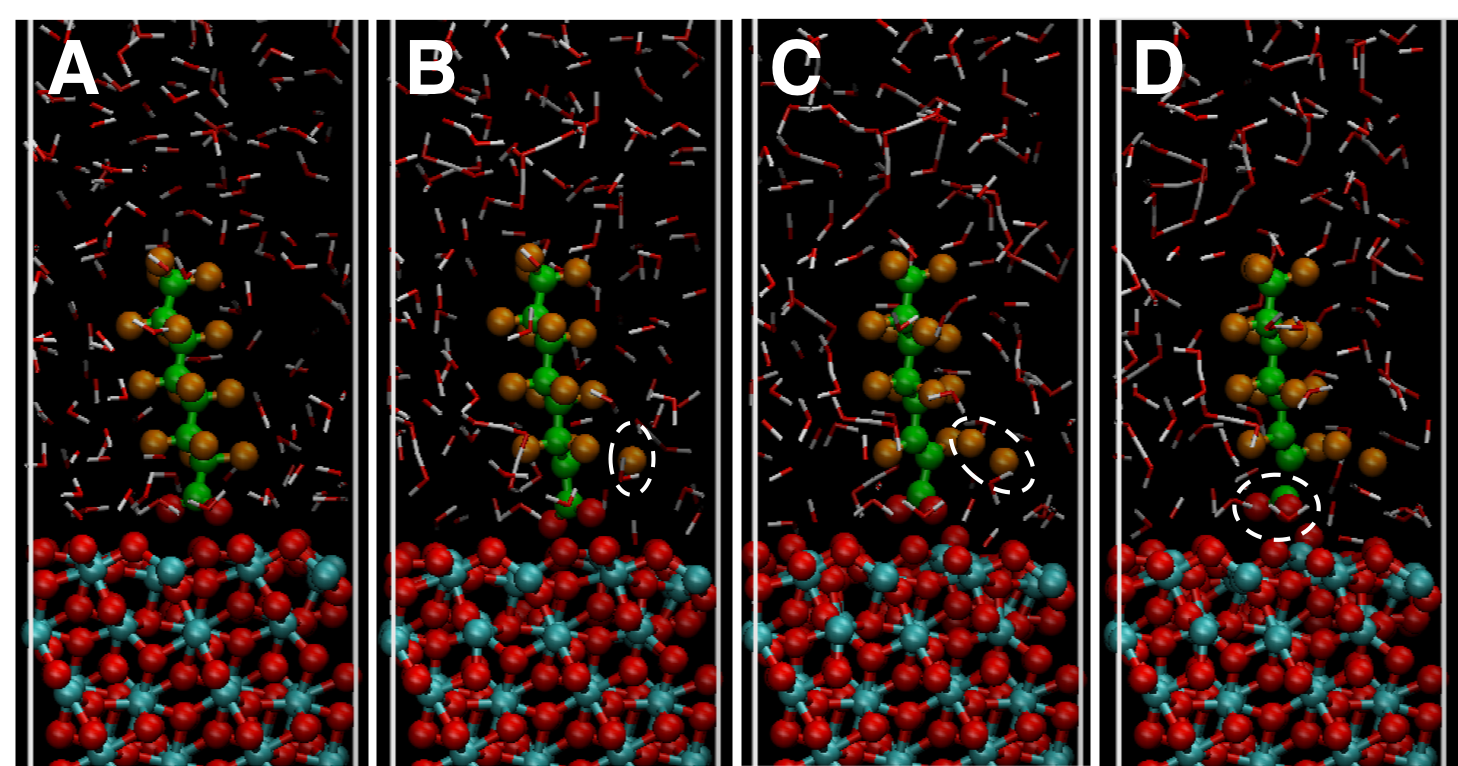
Figure 1. (A-D) Well equilibrated configuration (A), and reactive trajectories of defluorination (B,C), followed by decarboxylation (D) taken from a molecular dynamics simulation at 298 K at t=2, 2.001, 2.002, and 2.005 ns, respectively, illustrating the initial stage of the electrochemical oxidation of PFOA near a Ti4O7 electrode surface. Ti, O, C, F, and H atoms are color coded by cyan, red, green, orange, and white, respectively. Dashed ovals indicate cleaved atoms.


