Electrokinetic (Blue) Energy Conversion using external ion concentration polarization
Overview
Reverse Electrodialysis (RED) is a method for generating electric power from a difference in salinity between two water bodies (e.g. fresh water and salt water that may be found in close proximity near an estuary). Electrical voltage is generated due to the equilibration of chemical potential on differential diffusion of ions across a permselective membrane. The device converts the free energy in salinity gradients directly to electrical energy with no moving parts using only water as the raw material. Reverse Electrodialysis (and the related pressure retarded osmosis) are sometimes referred to as “Blue Energy” or “Osmotic Power” as power is generated through the mixing of fresh water with sea water. The concept of RED was first proposed in 1954 and implemented in a practical system more than two decades later. The technology has found its way into prototype power plants but its economic viability is yet to be demonstrated. The primary reason for this is the low power density, typically < 105 Watt per squared meter. When water is forced through narrow channels in materials such as silica, an electric potential difference (streaming potential) develops between the ends of the channel. The conversion of mechanical work to electrical energy results when counter-ions from the Debye layer shielding the charged walls are swept into the outlet reservoir thereby doing work against electrostatic forces. Though streaming potential has found some applications in niche areas (e.g. “Shoe Power”, a device embedded in walking shoes to convert mechanical energy to portable electrical power) it has not been used in commercial power generation. The reason for this is the extreme low measured efficiency of the energy conversion process, typically less than 13%.
Electrokinetic Energy Conversion (EEC) based power has obvious appeal from the point of view of cost and environmental impact. Indeed, in the original 1954 paper, it was pointed out “The osmotic pressure of sea water is about 20 atmospheres, so that when a river mixes with the sea, free energy equal to that obtainable from a waterfall 680 ft. high is lost.” Commercial development has however been thwarted by the very low efficiencies and power densities achieved to date. Here, we are testing an alternate approach that could circumvent the limitations of EEC by using an electrokinetic phenomenon known as Ion Concentration Polarization (ICP).
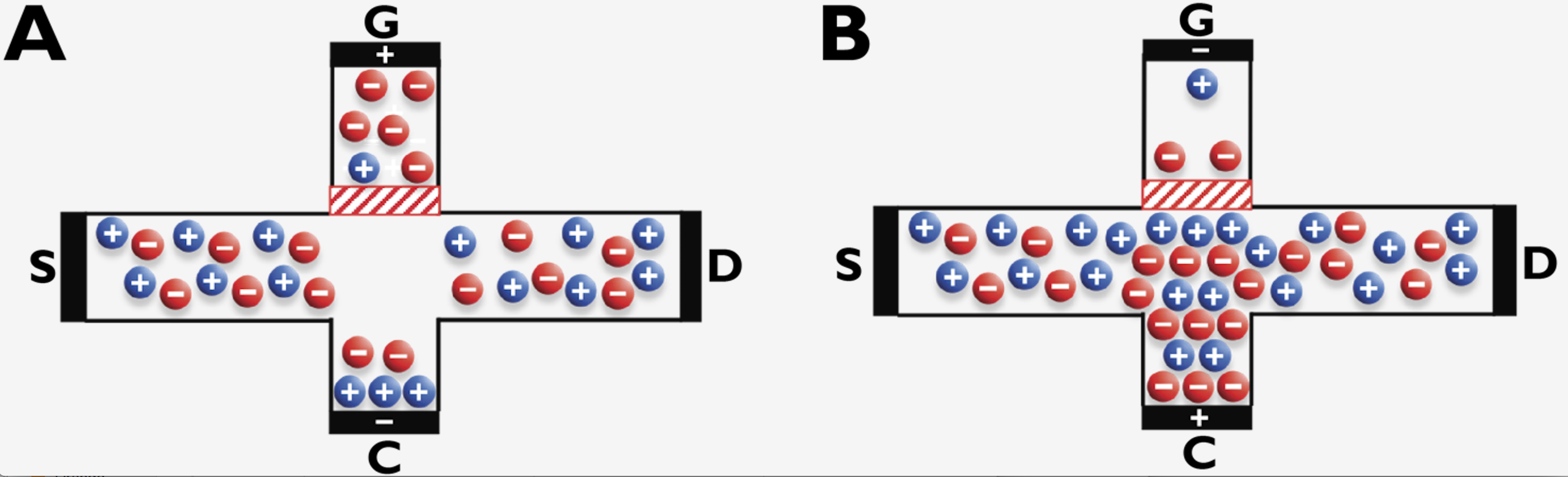
Figure 1. Schematic of the microfluidic anion exchange membrane hybrid device. A depletion zone under the anion exchange membrane (red hatched block) emerges when voltage at G is sufficiently higher than that under the membrane (A). Conversely, an enrichment zone under the membrane is generated when the polarities of G and C are reversed (B).


